Depth Perception Test
Alright, so you’re at MEPS to find out if you’re qualified to join. It’s nerve wracking to say the least. I know I worried about lots of things that I shouldn’t have. Here is my experience with the vision test, and a few pointers! Again, I joined the Air Force, so they require color blindness and depth perception tests as well as a regular eye exam.I waited a little while (like 5 minutes) and then went in to do the vision test. The man giving the exam had an eye patch and a big, gray beard. Everybody refers to him as ‘the pirate’ and he embraces it.Anyways, first thing was the color blindness test.
When I was at MEPS originally, I failed my depth perception test too. I then went to an outside doctor because I knew I had good depth perception. Sure enough, I passed. So now I get to go take the depth perception test again. I also learned through talking to a PJ in the Air Force that that test is hard, even for good depth perception. May 06, 2018 Published on May 6, 2018 This test will help you determine whether you have fully functional depth perception or not! You can do this online diagnostic to assess your binocular vision (or.
It’s just numbers made of dots and you tell them what number it is (Remember to speak clearly and confidently so that they can hear you). I probably had to do like 20 different ones, if not more. If you do fail, it isn’t necessarily disqualifying, it just affects the jobs that you will qualify for. Most females will be fine.
Men tend to have a lot more problems with this portion. I will attach an example image below.Next was just a normal vision test, You will do it with and then without your contacts/glasses. It’s on a machine, not the old school sign-on-the-wall way.
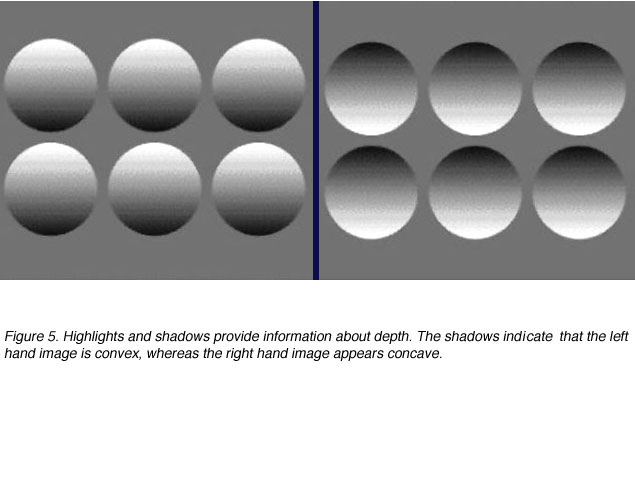
I think most people have done that before, and it’s no different at MEPS.You will also take a depth perception test if you are processing for the Air Force. The depth perception test was rows of five circles. There were several lettered sections, each section had three or four rows.
You will look at each row individually, and tell him which circle looks different. It will look closer, or like it’s popping out at you. See below for an example picture.but keep in mind that without the machine, all the circles look the same.That is almost exactly what it looked like when I took it.I will tell you a secret that I heard and that seemed to be accurate for me. I was told that of the five circles, it could not be the first or last circle, because it had to have one on both sides to compare it to. I never guessed either of those circles. The top row in each section was super obvious for me and the others got a little tricky. Another helpful hint, if you find yourself struggling, try using your peripherals.
Focus a little above the row or something, whatever works for you. Remember to take as much time as you need.That’s all I have about the vision portion, if you are headed to MEPS, good luck! Also if you have any other questions feel free to comment and I will get back to you. Thanks for reading! When I took the test with the five circles, they kept telling me that there was no “fifth” choice.
Then, when I was struggling, they told me “Does it look like anyone else is here?” and “Are we just going to stand there?”. Basically, it was pretty aggressive for a vision test. I don’t like how you said we can take our time in this article when clearly we can’t.Because of that confusing test, I am now having to see an eye consult based for Myopia and Astigmatism for a job that I want to be based on Computer Programming.
GreatNot sure why they treated me that way. Nobody else got the same treatment based on what I was seeing. Of course, they were tested by someone else. Hope it wasn’t biased. I was the only Asian applicant thereAt this point, I am just praying I get a waiver and that it is approved. I have been spending so much time preparing for the Air Force that I don’t know what to do if it doesn’t work out.Like.
Glaucoma is a leading cause of irreversible vision loss, and its pathological correlate is the loss of retinal ganglion cells and their axons. Current assessment of vision loss in glaucoma is performed in a standardised way with static perimetry that tests each eye separately.
There are also important visual functions that require interaction of inputs from both eyes; however, studies of binocular visual function in patients with glaucoma are limited in number and vary in their methodology., Among these functions, stereopsis, important for some daily activities, is a true binocular function that involves input from both eyes with the first integration at the level of the striate and extrastriate visual cortex.Two studies have examined stereopsis in glaucoma, and both reported stereopsis deficits in glaucoma patients. However, in glaucoma suspects, stereopsis deficits were detected in one study and not in the other. These studies used differing depth perception tests of dichoptically presented stimuli —namely, the random dot and line stereograms. Random dot patterns eliminate monocular cues to depth; however, the test requires fusion, and this can be abnormal in some people with otherwise normal stereopsis.
Line stereogram testing may allow depth to be inferred from monocular cues. In contrast with these “apparent” depth perception tests, the Frisby test of stereopsis presents real depth to the subject in the form of different plate thicknesses, such that the problems associated with dichoptically presented stimuli, including fusion, are avoided.
Here we investigate depth perception in glaucoma suspects using the Frisby test, and compare the results with age matched controls and glaucoma patients. Patients included in the study were examined by a glaucoma specialist. Patients were required to be between the ages of 40 years and 65 years, with no history of neurological disease, orthotropic by near and far cover‐uncover tests, with a minimum best corrected visual acuity of 20/30 in each eye, differing by no greater than one Snellen acuity line, and no history of incisional surgery within the previous 4 months. Primary open angle glaucoma was defined by characteristic glaucomatous optic nerve head findings and required the presence of corresponding visual field deficits in one or both eyes. Glaucoma suspects were required to have a normal Humphrey 24‐2 visual field and intraocular pressure (IOP) at least ⩾24 mm Hg or optic nerve head findings suspicious for glaucoma. All control subjects were age matched, with no incisional surgical history, and underwent a full eye examination.
Control subjects were recruited from hospital personnel or accompanying relatives of patients, and had normal eye examinations. One control subject had a history of laser peripheral iridotomy. Glaucoma suspects had no history of incisional ocular surgery, and two patients had a history of laser peripheral iridotomy.
Of the patients with glaucoma, nine had no history of eye surgery, five had cataract and trabeculectomy surgery in each eye, three patients had trabeculectomy surgery in one eye, and a single patient had cataract surgery in one eye. The interpupillary distance was measured for all patients. Stereoacuity measurementInformed consent was obtained from patients. Stereoscopic visual acuity was assessed using the Frisby stereotest (Clement Clarke International Ltd, Essex, UK) according to the instructions of the manufacturer. The Frisby stereotest consists of three plates of Perspex glass measuring 1 mm, 3 mm, and 6 mm in thickness. This test was performed for all patients under the same conditions, and subjects had no previous experience with this type of test. Individuals performing the testing were not aware of the diagnosis.
The subject's head was placed on a chin rest to prevent head movement, and plates of varying thickness were shown perpendicular to the visual axis at several viewing distances taking care to avoid plate movement. The patient was instructed to close his/her eyes before testing and between plate presentations. The subject was asked to report which of the four quadrants contained the circle in depth, and stereoacuities were recorded with a range of values from 20 seconds of arc to 340 seconds of arc. The lowest disparity which the patient can reliably discriminate was recorded and this stereothreshold was a measure of stereoacuity.The 6 mm plate was shown at a viewing distance of 80 cm. If the answer was correct, the 3 mm plate was presented at the same distance.
If the answer was incorrect for the 3 mm plate, a score of 85 seconds of arc was recorded. If the answer was correct, the 1 mm plate was presented. If the answer was incorrect, a score of 40 seconds of arc was recorded, and if the answer was correct, a score of 20 seconds of arc was recorded.If the answer was incorrect for the 6 mm plate at 80 cm, the same plate was viewed at a distance of 60 cm.
If the answer was correct, a score of 150 seconds of arc was recorded; while if the answer was incorrect, the 6 mm plate was presented at 40 cm. If the answer was correct, a score of 340 seconds of arc was recorded. If the subject could not detect the quadrant with depth effect at this distance, the highest stereothreshold value tested (340 seconds of arc) was assigned for statistical analysis. Statistical analysisAll data were tested for normality using software (GraphPad, San Diego, CA, USA).
For parameters with Gaussian distributions such as inter‐pupillary distance, visual acuity, and age, the ANOVA test was used to make comparisons among the three groups. If there was a significant difference among groups, Bonferroni's multiple comparison tests were used to compare glaucoma patients to normals and suspects to normals. For the stereovision threshold which does not show Gaussian distribution, the non‐parametric Kruskall‐Wallis test was used to compare stereothreshold values among the three groups (glaucoma patients, suspects, and normals), followed by Dunn's multiple comparison tests used to compare glaucoma patients with normals, and suspects with normals. Data are presented as mean (SE).
ResultsNo differences in interpupillary distance, age, or visual acuity were found among the three groups (p0.05) (table 1). There was a significant difference between the mean cup/disc ratio between controls and suspects and controls and glaucoma (p.
Figure 1 Mean stereothreshold in seconds of arc (SE) for control (n = 19), suspect (n = 16), and glaucoma (n = 18) subjects.The mean stereothreshold of the glaucoma and age matched control groups were 148.1 (SE 33.8) seconds of arc and 26.6 (3.7) seconds of arc, respectively. The mean stereothreshold in glaucoma patients was significantly higher compared to controls (p = 0.0004) (fig 1).Figure 2 shows the frequency distribution of stereoacuity measurements across the three groups. Ninety five per cent of control subjects showed stereothresholds between 20–40 seconds of arc, compared to 44% of patients in both suspect and glaucoma groups. Stereothresholds of ⩾340 seconds of arc were seen only in suspect (31%) and glaucoma patients (33%).
Can you complete different tasks as one, and grow a family?From forests to beaches, a vast open-world is waiting for you. Ultimate wolf simulator.
DiscussionIn this study, depth perception in control subjects using the Frisby test was similar to results obtained using other depth perception tests. The mean stereothreshold of 27 seconds of arc observed in control subjects was similar to mean stereothresholds of 16 and 27 seconds of arc observed in control subjects between 20 years and 50 years, and between 60 and 70 years, respectively.This study confirms that stereopsis is reduced in glaucoma using a different test of real depth perception., In fact, our finding of stereoacuity of 148 seconds of arc is similar to the 148 seconds of arc reported for glaucoma patients by Bassi and Galanis. Furthermore, reduced stereopsis in our glaucoma patients was found in a relatively younger group with mean age of 55 years compared to 62 years and 63 years. This finding supports that impaired depth perception seen in glaucoma patients is related to the disease process rather than age related visual dysfunction.We observed significantly reduced stereopsis in glaucoma suspects, with a mean stereothreshold of 144 seconds of arc, and this is in keeping with the 118 seconds of arc reported by Essock and co‐workers.

This effect was seen in our study in a younger group of glaucoma suspects with mean age of 57 years compared to 61 years. It is not clear why stereopsis deficits were not observed in glaucoma suspects in another paper in which suspects had significantly higher intraocular pressure and cup to disc ratios compared to controls. It is possible that the random dot stereogram test used in that study and known to lend itself to monocular cues, may have underestimated any decreased depth perception in the glaucoma suspects.We obtained similar results of reduced stereoacuity in both suspect and glaucoma groups, and this was not related to reduced visual acuity as the visual acuities of suspect and glaucoma were similar to that seen in normals. The Frisby test is presented to the subject in the form of different plate thicknesses with great care to avoid head and plate movement, such that the problems associated with dichoptically presented stimuli are avoided. The finding of reduced depth perception in glaucoma suspects suggests glaucomatous disease and related pre‐perimetric visual dysfunction. We cannot exclude the possibility that a non‐glaucomatous process is also involved.
Further studies of a larger sample size with reduced standard deviation may help to better understand impaired depth perception in glaucoma suspects.The neuronal basis of stereovision depends on disparity cells sensitive to binocular disparity, located in the primary visual cortex and extrastriate areas. Marked stereovision deficits in glaucoma suspects with normal achromatic visual fields suggest disrupted binocular vision activities. The causes of profound disruption of stereoacuity in suspects and glaucoma patients are not yet known. Spatial sampling array disorder at the retinal ganglion cell level has been previously proposed. There is evidence that the neuropathology of glaucoma extends beyond retinal ganglion cells to include geniculo‐cortical pathways in experimental monkey, and human glaucoma. We suggest the additional interpretation that loss of stereoacuity in glaucoma suspects and glaucoma may be the result of the relative delay of input from one eye compared to input from the other eye, as seen with cortical visual evoked potentials in glaucoma suspects and patients., This may affect binocular interactions taking place at the level of the visual cortex, where neural degeneration in human glaucoma has been observed.
Further studies of binocular functions are needed to understand visual dysfunction in glaucoma suspects and may be helpful in uncovering early disease.